-
 Femara Aaster Health and Sports Solutions
1 × £83.00
Femara Aaster Health and Sports Solutions
1 × £83.00 -
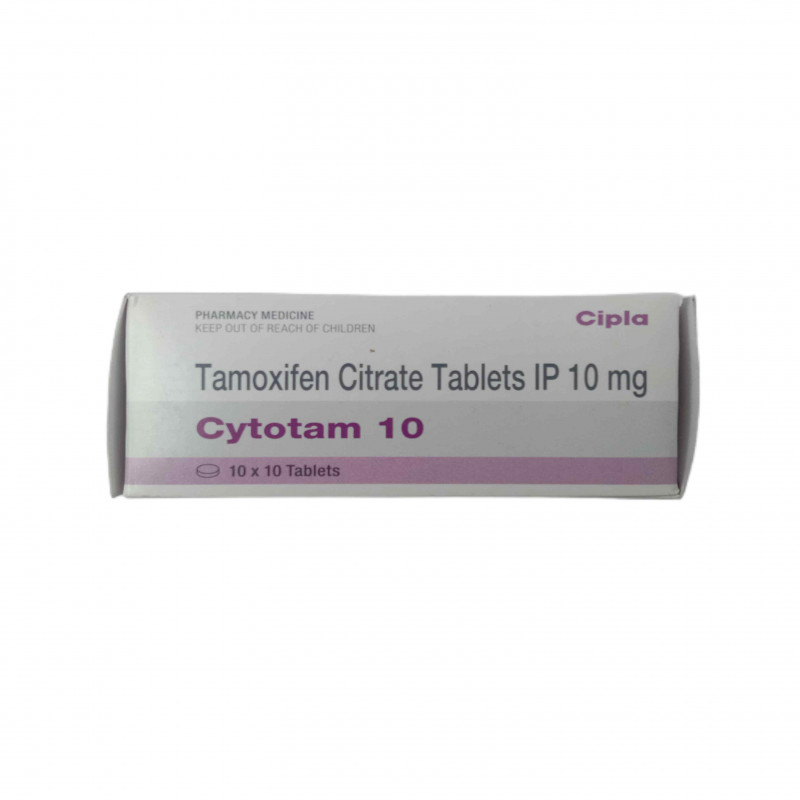
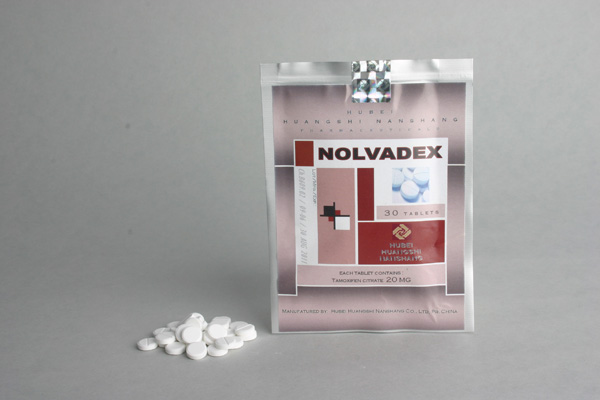
 Tamoxifen 20 Aaster Health and Sports Solutions
1 × £46.00
Tamoxifen 20 Aaster Health and Sports Solutions
1 × £46.00 -
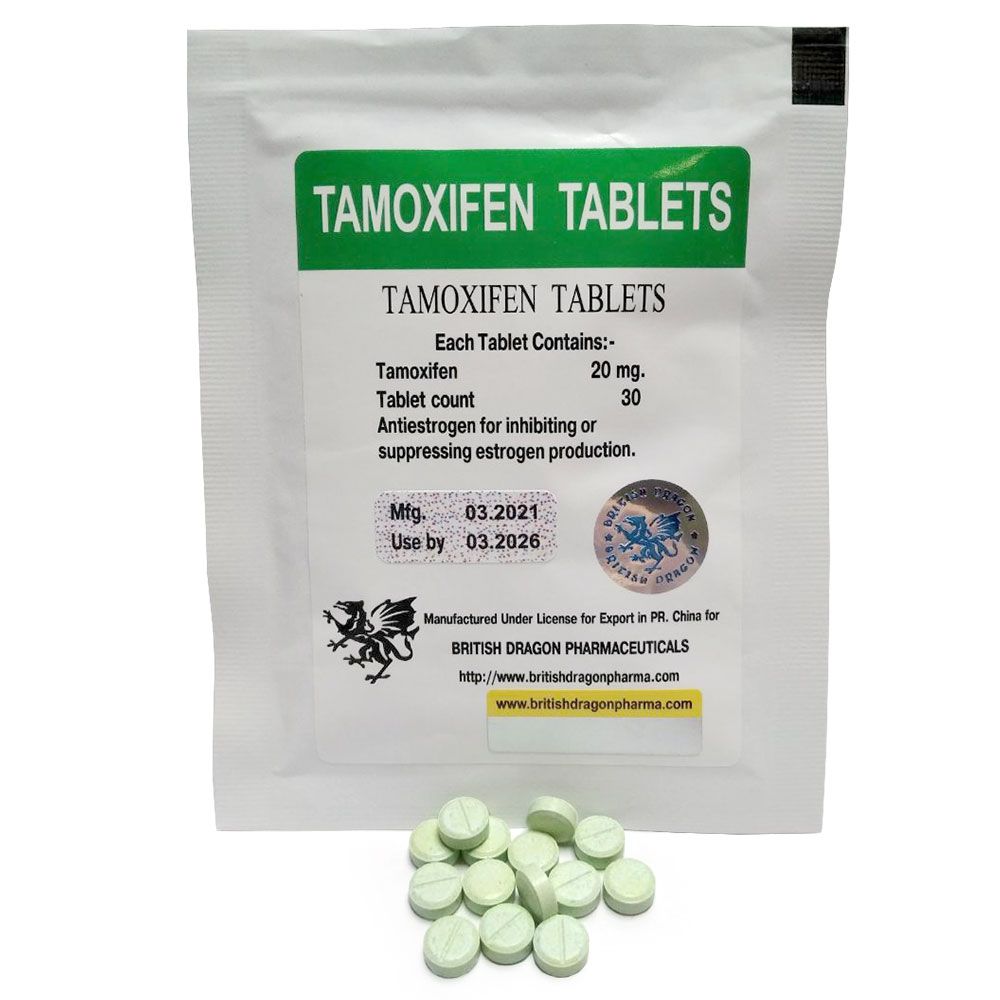
 Tamoxifen 20 mg Ice Pharmaceuticals
1 × £52.00
Tamoxifen 20 mg Ice Pharmaceuticals
1 × £52.00
Subtotal: £181.00

Reviews
There are no reviews yet.